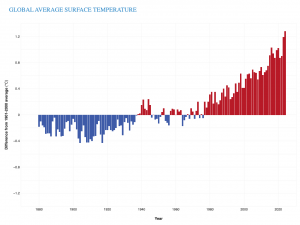
Last Friday was the 81st anniversary of the Allied invasion of Europe that began with the landings on the beaches at Normandy. The combined land, air, and sea assault of June 6, 1944 remains the largest such event in history. The success of the invasion was extraordinarily dependent of weather conditions. More than three months before the invasion, a combined British and American forecasting team began rigorous forecast exercises designed to iron out the physical and logistical kinks of such a coordinated effort. As June drew near, the nature of this collaboration was still problematic as the two groups employed vastly different methods in fashioning the requisite 3-5 days forecasts – at the time, absolutely primitive in the underlying science as compared to what is possible at such ranges today. The British were attempting to make such forecasts based upon the understanding of atmospheric dynamics that had grown substantially during the war. The Americans were employing a method based on a statistically- based search through old weather data for historical analogues that could be used to guide the forecast.
To maintain secrecy, a large portion of the Allied fleet was squirreled far away in northern Scotland. Consequently, 5 days of lead time was required to mobilize these forces. Thus, General Eisenhower needed to know by May 31 whether the first week of June, the prospective target for the invasion, would provide favorable weather. The forecasters foresaw a break in that year’s unusually stormy late spring and suggested June 5 would work. As the day approached, the team realized that a one-day postponement would offer better conditions prompting Eisenhower to make the fateful decision to invade on June 6, under barely acceptable conditions. Had the Allies delayed, the combination of lunar cycle, tides and weather almost certainly would have postponed the invasion for more than a month likely costing the effort the tremendous advantage of secrecy.
Steve Ackerman and Jonathan Martin, professors in the UW-Madison department of atmospheric and oceanic sciences, are guests on WHA radio (970 AM) at noon the last Monday of each month. Send them your questions at stevea@ssec.wisc.edu or jemarti1@wisc.edu.