Ozone is a colorless gas made up of three oxygen atoms (chemically denoted as O3). It occurs naturally in small amounts in the upper atmosphere (the stratosphere), about 18 miles above the surface.
Ozone in the stratosphere is a result of a balance between sunlight that creates ozone and chemical reactions that destroy it. Ozone is created when oxygen (O2), is split apart by ultraviolet energy emitted by the sun into single oxygen atoms. The single oxygen atoms can rejoin to make O2, or they can join with O2 molecules to make ozone.
The winter atmosphere above Antarctica is very cold. These extremely cold temperatures are a good environment for forming polar stratospheric clouds, or PSC. PSCs begin to form during June, which is wintertime at the South Pole. Chemicals on the surface of the particles composing PSCs result in chemical reactions that remove atmospheric chlorine.
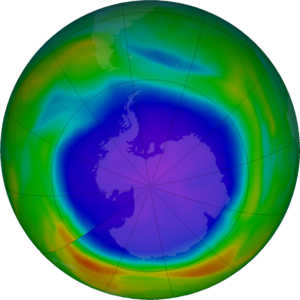
When the Sun returns to the Antarctic stratosphere in the spring (our fall), sunlight splits the chlorine molecules into highly reactive chlorine atoms that rapidly deplete ozone. The depletion is so rapid that it has been termed a “hole in the ozone layer.”
The amount of ozone in the atmosphere is routinely measured from satellites. The ozone hole appears high over the continent of Antarctica as very low values of ozone in the stratosphere. Typically, the Antarctic ozone hole has its largest area in early September and lowest values in late September to early October. This year’s hole is shaping up to be larger than average in area, but well within expectations.
Sept. 16 is the International Day for the Preservation of the Ozone Layer. That day celebrates the 1987 anniversary of the Montreal Protocol on Substances that Deplete the Ozone Layer. The Montreal Protocol led to a ban on a group of chemicals called halocarbons that were blamed for exacerbating the annual ozone hole.
While the ozone layer is beginning to recover, it is likely to take until the 2060s for the ozone-depleting substances used in refrigerants and spray cans to completely disappear from the atmosphere.
Steve Ackerman and Jonathan Martin, professors in the UW-Madison department of atmospheric and oceanic sciences, are guests on WHA radio (970 AM) at 11:45 a.m. the last Monday of each month. Send them your questions at stevea@ssec.wisc.edu or jemarti1@wisc.edu.

